Ophthalmologic Laboratory-Clinics
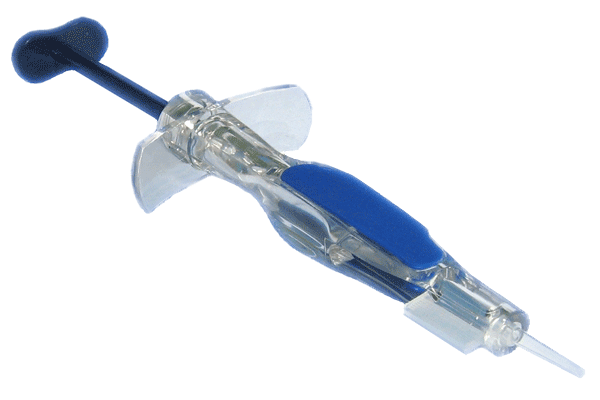
Ophthalmic injectors, manufactured by OLC "US OPTICS", are designed for implantation of acrylic one-component hydrophilic and hydrophobic (soft) intraocular lenses (HIOL) into the human eye through a cut over the iris to the center of the eye.
The delivery scope of the injector consists of an injector with a silicone tip and a cartridge.
Each ophthalmic injector is sealed in an individual bag of material intended for sterilization with ethylene oxide, embedded in a box, which is sealed in a polymer film.
Qualitative characteristics:
- the excellent ergonomics of the product provides the convenience of control over the process of implantation of HIOL into the capsular bag;
- the injector allows implantation of the HIOL through an operative incision of 1.8 mm in size.
The consumer (sterilization) package contains a label with the marking on which:
- bar-code;
- serial number (for example, № 150100500 means: 15 - year of sterilization (2015), 01 - month of sterilization (January), 00500 - products unit number (500));
- external diameter of cartridge tip.

In the delivery state the product is sterile (sterilized by gas sterilization in an ethylene oxide medium). Shelf life is 36 months from the date of sterilization.

| Cartridge model | External diameter of cartridge tip, mm | Diameter of the outlet, mm | Length of tip, mm | Total Cartridge Length L, mm |
| 18-С | 1.8 ± 0.2 | 1.35 ± 0.2 | 14.5 ± 2.0 | 34.0 ± 2.0 |
| 20-С | 2.0 ± 0.2 | 1.39 ± 0.2 | ||
| 22-С | 2.2 ± 0.2 | 1.60 ± 0.2 | ||
| 24-С | 2.4 ± 0.2 | 1.90 ± 0.2 | ||
| 26-С | 2.6 ± 0.2 | 2.20 ± 0.2 |
Implantation of HIOL can be performed only by ophthalmologists of appropriate qualification, who have completed training in mastering the technique of implantation.
-
US Optics - winner of "Best National Product of the Year"
US Optics - winner of "Best National Product of the Year 2006"
Nomination: Design and manufacture of medical devices, equipment and materials. -
Who is who. Ukraine medical 2006
Ophthalmologic Laboratory-Clinics "US Optics" - Chairman of the Board Anatoly Mushtuk. -
US Optics - "first aid" of national ophthalmology
October 10, 2014 Ophthalmologic Laboratory-Clinics "US OPTICS" celebrates its 20th anniversary.